Variable radioactive decay
Moderator: Alyrium Denryle
- montypython
- Jedi Master
- Posts: 1130
- Joined: 2004-11-30 03:08am
Variable radioactive decay
Is it conceivable to change the radioactive decay rate for a given particle by manipulating its quantum stability and/or nuclear forces? The links I've seen on Google are often linked to creationists and associated sites, so I just wanted to ask the question without that type of BS attached.
- SilverWingedSeraph
- Jedi Knight
- Posts: 965
- Joined: 2007-02-15 11:56am
- Location: Tasmania, Australia
- Contact:
Re: Variable radioactive decay
A lot of Creationists seem to like saying that "studies have shown that radioactive decay rates can be altered in the lab by a factor of [insert stupidly large and arbitrary number here]". Near as I can tell, it's outright bullshit. If there were any known way of significantly altering radioactive decay rates, radioactive waste wouldn't be a problem, near as I can tell. But then, my skills with science are limited mostly to what I've learned off the internet, so you're probably better off waiting for one of the more scientifically savvy members of the board to comment.
/l、
゙(゚、 。 7
l、゙ ~ヽ
じしf_, )ノ
゙(゚、 。 7
l、゙ ~ヽ
じしf_, )ノ
- Zixinus
- Emperor's Hand
- Posts: 6663
- Joined: 2007-06-19 12:48pm
- Location: In Seth the Blitzspear
- Contact:
Re: Variable radioactive decay
Short answer: It's pure bullshit.
Long answer:
As far as I know, you radioactive decay does rate not change on its own, with either atoms or particles. Technically, I recall that particles don't decay?
The only thing that comes to mind is half-life. The only way to reduce the half-life of a given quantity of an radioactive element in by transforming it into another element with neutron bombardment. From Element XY- 245 that has a decay rate of 200 years you can turn it into Element XY - 246 that decays under 20 days. With lower decay rate, comes much more radiation though.
An example is Uranium 238. It's half-life is measured in million of years if I recall. Turn one of its neutrons into a proton right and you'll get Plutonium 238 that decays under 82 years. Pu-238's decay is so powerful that it can be used as an instant heat-source, look up RTG's.
However, this is a the process of making an unstable particle even more unstable by outside force. It's like hitting a crumbling building with a RPG: the building is already crumbling.
Long answer:
As far as I know, you radioactive decay does rate not change on its own, with either atoms or particles. Technically, I recall that particles don't decay?
The only thing that comes to mind is half-life. The only way to reduce the half-life of a given quantity of an radioactive element in by transforming it into another element with neutron bombardment. From Element XY- 245 that has a decay rate of 200 years you can turn it into Element XY - 246 that decays under 20 days. With lower decay rate, comes much more radiation though.
An example is Uranium 238. It's half-life is measured in million of years if I recall. Turn one of its neutrons into a proton right and you'll get Plutonium 238 that decays under 82 years. Pu-238's decay is so powerful that it can be used as an instant heat-source, look up RTG's.
However, this is a the process of making an unstable particle even more unstable by outside force. It's like hitting a crumbling building with a RPG: the building is already crumbling.
Credo!
Chat with me on Skype if you want to talk about writing, ideas or if you want a test-reader! PM for address.
Chat with me on Skype if you want to talk about writing, ideas or if you want a test-reader! PM for address.
- montypython
- Jedi Master
- Posts: 1130
- Joined: 2004-11-30 03:08am
Re: Variable radioactive decay
I do remember in physics class about particles such as electrons and protons have the lifespan of the Universe itself, so that wouldn't be an issue of contention. The concept was regarding the rates of decay for elements in terms of energy and particle release to achieve a stable state, like U-238 decaying into lead. Now neutrons can undergo decay to transmute into a proton, electron and neutrino, but if one wanted to manipulate U-238 into conventional Pb by causing the surplus energy state to be discharged as photonic and particle energy in a single impulse, would that work?Zixinus wrote:Short answer: It's pure bullshit.
Long answer:
As far as I know, you radioactive decay does rate not change on its own, with either atoms or particles. Technically, I recall that particles don't decay?
The only thing that comes to mind is half-life. The only way to reduce the half-life of a given quantity of an radioactive element in by transforming it into another element with neutron bombardment. From Element XY- 245 that has a decay rate of 200 years you can turn it into Element XY - 246 that decays under 20 days. With lower decay rate, comes much more radiation though.
An example is Uranium 238. It's half-life is measured in million of years if I recall. Turn one of its neutrons into a proton right and you'll get Plutonium 238 that decays under 82 years. Pu-238's decay is so powerful that it can be used as an instant heat-source, look up RTG's.
However, this is a the process of making an unstable particle even more unstable by outside force. It's like hitting a crumbling building with a RPG: the building is already crumbling.
- montypython
- Jedi Master
- Posts: 1130
- Joined: 2004-11-30 03:08am
Re: Variable radioactive decay
Ghetto edit: Reason for asking the previous question regarding rapid energy discharge for decay stabilization was for a story idea.
- GrandMasterTerwynn
- Emperor's Hand
- Posts: 6787
- Joined: 2002-07-29 06:14pm
- Location: Somewhere on Earth.
Re: Variable radioactive decay
As radioactive decay is governed by the strong and weak nuclear forces . . . which are fundamental interactions of spacetime . . . no, can't be done, not without breaking the universe.montypython wrote:Is it conceivable to change the radioactive decay rate for a given particle by manipulating its quantum stability and/or nuclear forces? The links I've seen on Google are often linked to creationists and associated sites, so I just wanted to ask the question without that type of BS attached.
As for the question you posed later in the thread, there is a vanishingly small probability that a given atom of U-238 will undergo the whole decay chain to Pb in very short order. Radioactive decay is a probabilistic process. To transmute U-238 into lead like that would require that you magically control probability. But that goes back to that whole breaking the universe thing.
Tales of the Known Worlds:
2070s - The Seventy-Niners ... 3500s - Fair as Death ... 4900s - Against Improbable Odds V 1.0
2070s - The Seventy-Niners ... 3500s - Fair as Death ... 4900s - Against Improbable Odds V 1.0
- SpacedTeddyBear
- Jedi Master
- Posts: 1093
- Joined: 2002-08-20 11:54pm
- Location: San Jose, Ca
Re: Variable radioactive decay
I believe that process is called fission.montypython wrote:Now neutrons can undergo decay to transmute into a proton, electron and neutrino, but if one wanted to manipulate U-238 into conventional Pb by causing the surplus energy state to be discharged as photonic and particle energy in a single impulse, would that work?
-
Lord of the Abyss
- Village Idiot
- Posts: 4046
- Joined: 2005-06-15 12:21am
- Location: The Abyss
Re: Variable radioactive decay
Yes, it's been done. I think that this article mentions it, but don't want to buy it to find out.montypython wrote:Is it conceivable to change the radioactive decay rate for a given particle by manipulating its quantum stability and/or nuclear forces?
I read an article on the subject long ago, but can't find a link. As I recall, the idea is that you use a laser to "interrogate" the radioactive atoms in question; this collapses their quantum state and prevents them from having the quantum certainty that at least some atoms need to decay. Note that this can only work on gases and surfaces, since the laser has to hit the atoms.Physical Review wrote:G. C. Baldwin and S. A. Wender
Los Alamos National Laboratory, Los Alamos, New Mexico 87545
Received 15 March 1982
Optical excitation of an atom containing an isomeric nucleus should affect its internal conversion coefficient through modification of its bound-state electronic wave functions as well as by interaction with the outgoing electron. The extreme case of 235mU, which decays entirely by internal conversion and for which pronounced environmental effects on the half-life have been observed, is considered. Resonant (bound-state) effects on the lifetime and electron emission spectrum are predicted to occur with moderate-power cw laser irradiation.
©1982 The American Physical Society
"There are two novels that can change a bookish fourteen-year old's life: The Lord of the Rings and Atlas Shrugged. One is a childish fantasy that often engenders a lifelong obsession with its unbelievable heroes, leading to an emotionally stunted, socially crippled adulthood, unable to deal with the real world. The other, of course, involves orcs." - John Rogers
-
Lord of the Abyss
- Village Idiot
- Posts: 4046
- Joined: 2005-06-15 12:21am
- Location: The Abyss
Re: Variable radioactive decay
Gah; make that UNcertainty.Lord of the Abyss wrote: this collapses their quantum state and prevents them from having the quantum certainty
"There are two novels that can change a bookish fourteen-year old's life: The Lord of the Rings and Atlas Shrugged. One is a childish fantasy that often engenders a lifelong obsession with its unbelievable heroes, leading to an emotionally stunted, socially crippled adulthood, unable to deal with the real world. The other, of course, involves orcs." - John Rogers
- Zixinus
- Emperor's Hand
- Posts: 6663
- Joined: 2007-06-19 12:48pm
- Location: In Seth the Blitzspear
- Contact:
Re: Variable radioactive decay
Radioactive decay IS discharging energy in the form of photonic and particle energy.I do remember in physics class about particles such as electrons and protons have the lifespan of the Universe itself, so that wouldn't be an issue of contention. The concept was regarding the rates of decay for elements in terms of energy and particle release to achieve a stable state, like U-238 decaying into lead. Now neutrons can undergo decay to transmute into a proton, electron and neutrino, but if one wanted to manipulate U-238 into conventional Pb by causing the surplus energy state to be discharged as photonic and particle energy in a single impulse, would that work?
As in a single impulse? Ermm, the only nuclear reaction I know of that could do that would anti-matter - matter reactions. Otherwise the only other reaction that I know where you get from a high energy state to a small one is called fission. Even then, its gradual somewhat, U-235 will not turn into pure H+ ions but into two, still heavy and radioactive atoms.
Credo!
Chat with me on Skype if you want to talk about writing, ideas or if you want a test-reader! PM for address.
Chat with me on Skype if you want to talk about writing, ideas or if you want a test-reader! PM for address.
- Ariphaos
- Jedi Council Member
- Posts: 1739
- Joined: 2005-10-21 02:48am
- Location: Twin Cities, MN, USA
- Contact:
Re: Variable radioactive decay
I recall reading something about 'a watched particle doesn't decay' and ever since I've been trying to -find- that article again...
Give fire to a man, and he will be warm for a day.
Set him on fire, and he will be warm for life.
Set him on fire, and he will be warm for life.
Re: Variable radioactive decay
I did a question on that is my QM undergrad course. I cant remember the exact details, and i dont have my notes here unfortunately, but the gist of it was that with a 2 state quantum system if you keep prodding it then the wavefunction gets reset to the original state rather than jumping/evolving to the next one as it would if you didnt interfere. The conclusion was that a watched quantum kettle never boils.Xeriar wrote:I recall reading something about 'a watched particle doesn't decay' and ever since I've been trying to -find- that article again...
Apparently nobody can see you without a signature.
Re: Variable radioactive decay
Actually the question is still on the web, its Question 8 on this sheet. This is the easy introductory QM course, so the examples are generally pretty easy to follow and guide you to the necessary steps.
Apparently nobody can see you without a signature.
- Ariphaos
- Jedi Council Member
- Posts: 1739
- Joined: 2005-10-21 02:48am
- Location: Twin Cities, MN, USA
- Contact:
Re: Variable radioactive decay
Awesome, thanks!
I've occasionally had mental images of several trillion atoms of some volatile isotope getting trapped and preserved for testing.
I've occasionally had mental images of several trillion atoms of some volatile isotope getting trapped and preserved for testing.
Give fire to a man, and he will be warm for a day.
Set him on fire, and he will be warm for life.
Set him on fire, and he will be warm for life.
- Gil Hamilton
- Tipsy Space Birdie
- Posts: 12962
- Joined: 2002-07-04 05:47pm
- Contact:
Re: Variable radioactive decay
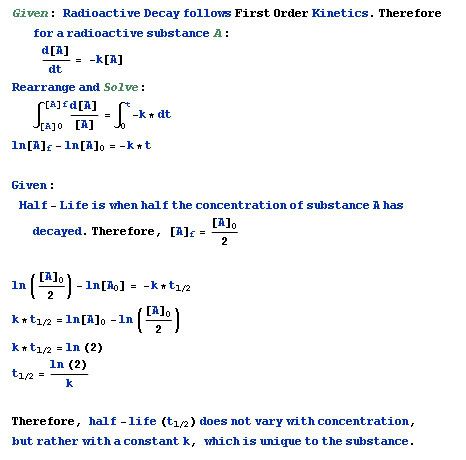
Typed up in Mathematica. Radioactive decay is a constant for a substance. Booyah.
"Show me an angel and I will paint you one." - Gustav Courbet
"Quetzalcoatl, plumed serpent of the Aztecs... you are a pussy." - Stephen Colbert
"Really, I'm jealous of how much smarter than me he is. I'm not an expert on anything and he's an expert on things he knows nothing about." - Me, concerning a bullshitter
"Quetzalcoatl, plumed serpent of the Aztecs... you are a pussy." - Stephen Colbert
"Really, I'm jealous of how much smarter than me he is. I'm not an expert on anything and he's an expert on things he knows nothing about." - Me, concerning a bullshitter
Re: Variable radioactive decay
Gil, aren't you assuming to start with that k is constant? That's the decay rate, and since the question at hand is whether the decay rate can change, you're begging the question.
A Government founded upon justice, and recognizing the equal rights of all men; claiming higher authority for existence, or sanction for its laws, that nature, reason, and the regularly ascertained will of the people; steadily refusing to put its sword and purse in the service of any religious creed or family is a standing offense to most of the Governments of the world, and to some narrow and bigoted people among ourselves.
F. Douglass
- Gil Hamilton
- Tipsy Space Birdie
- Posts: 12962
- Joined: 2002-07-04 05:47pm
- Contact:
Re: Variable radioactive decay
k has to be constant in order for it to be first order kinetics in the first place. It's a rate constant, it doesn't change by definition. The point is that when you solve the differential equation, any references to variables disappear. Thus, the decay rate can't change for a given substance.Surlethe wrote:Gil, aren't you assuming to start with that k is constant? That's the decay rate, and since the question at hand is whether the decay rate can change, you're begging the question.
I suppose you could question whether or not radioactivity follows first order kinetics or not, though.
"Show me an angel and I will paint you one." - Gustav Courbet
"Quetzalcoatl, plumed serpent of the Aztecs... you are a pussy." - Stephen Colbert
"Really, I'm jealous of how much smarter than me he is. I'm not an expert on anything and he's an expert on things he knows nothing about." - Me, concerning a bullshitter
"Quetzalcoatl, plumed serpent of the Aztecs... you are a pussy." - Stephen Colbert
"Really, I'm jealous of how much smarter than me he is. I'm not an expert on anything and he's an expert on things he knows nothing about." - Me, concerning a bullshitter
Re: Variable radioactive decay
I think the point is that while there are also chemical reactions that follow first order kinetics, when you dump a catalyst into the mix the rate changes significantly. While the new rate may still be following the first order structure (although possibly not with the different mechanism), the rate has changed significantly. (I seem to recall you can catalyse the decomposition of hydrogen peroxide with something, but that might not be a first order rates thing to begin with, and might not be after the catalysation... not terribly convincing, but that might be an example of what i'm talking about, and i'm sure there are others that someone who likes chemistry can trot out)Gil Hamilton wrote:k has to be constant in order for it to be first order kinetics in the first place. It's a rate constant, it doesn't change by definition. The point is that when you solve the differential equation, any references to variables disappear. Thus, the decay rate can't change for a given substance.Surlethe wrote:Gil, aren't you assuming to start with that k is constant? That's the decay rate, and since the question at hand is whether the decay rate can change, you're begging the question.
I suppose you could question whether or not radioactivity follows first order kinetics or not, though.
What you actually want to do is assume k=k(t) and then keep the right hand side in integral form, and then you dont just get everything to cancel, you have an integral equation which has determined [in this case just the time till half the stuff is gone] t_1/2 [as long as k(t) doesnt do anything silly] and wont have a proper half life.
I mean for the example of a catalysed reaction where catalyst is added at time T you would have k defined as
k= k1 t<T
k2 t>=T
Apparently nobody can see you without a signature.
- Gil Hamilton
- Tipsy Space Birdie
- Posts: 12962
- Joined: 2002-07-04 05:47pm
- Contact:
Re: Variable radioactive decay
Except I would turn around and trot out that catalysts work by lowering the activation energy of a reaction by providing a different path than the original reaction. It's not actually speeding up a reaction, that's a misconception based on the layman definition of what a catalyst does, but provides a more energy efficient different means for A to get to B.Steel wrote:I think the point is that while there are also chemical reactions that follow first order kinetics, when you dump a catalyst into the mix the rate changes significantly. While the new rate may still be following the first order structure (although possibly not with the different mechanism), the rate has changed significantly. (I seem to recall you can catalyse the decomposition of hydrogen peroxide with something, but that might not be a first order rates thing to begin with, and might not be after the catalysation... not terribly convincing, but that might be an example of what i'm talking about, and i'm sure there are others that someone who likes chemistry can trot out)
However, more to the point, catalysts are somewhat irrelevant to radioactive decay, aren't they?
"Show me an angel and I will paint you one." - Gustav Courbet
"Quetzalcoatl, plumed serpent of the Aztecs... you are a pussy." - Stephen Colbert
"Really, I'm jealous of how much smarter than me he is. I'm not an expert on anything and he's an expert on things he knows nothing about." - Me, concerning a bullshitter
"Quetzalcoatl, plumed serpent of the Aztecs... you are a pussy." - Stephen Colbert
"Really, I'm jealous of how much smarter than me he is. I'm not an expert on anything and he's an expert on things he knows nothing about." - Me, concerning a bullshitter
- Gil Hamilton
- Tipsy Space Birdie
- Posts: 12962
- Joined: 2002-07-04 05:47pm
- Contact:
Re: Variable radioactive decay
I suppose in hindsight I should stop, since I automatically in my brain pencilled in "constant, given specific conditions" in the case of chemical reactions. The rate constant for a chemical reaction has a temperature dependance baked into it, for example, so I suppose if you only cared about [A] -> , without pencilling in any conditions (which seems a very odd thing to do from my perspective), then rates of reaction can be variable.
However, I still don't see what this has to do with radioactive decay.
However, I still don't see what this has to do with radioactive decay.
"Show me an angel and I will paint you one." - Gustav Courbet
"Quetzalcoatl, plumed serpent of the Aztecs... you are a pussy." - Stephen Colbert
"Really, I'm jealous of how much smarter than me he is. I'm not an expert on anything and he's an expert on things he knows nothing about." - Me, concerning a bullshitter
"Quetzalcoatl, plumed serpent of the Aztecs... you are a pussy." - Stephen Colbert
"Really, I'm jealous of how much smarter than me he is. I'm not an expert on anything and he's an expert on things he knows nothing about." - Me, concerning a bullshitter
- Kuroneko
- Jedi Council Member
- Posts: 2469
- Joined: 2003-03-13 03:10am
- Location: Fréchet space
- Contact:
Re: Variable radioactive decay
You're missing the point. By assuming first-order kinetics in the first place, you beg the question, because the form has a constant rate by definition. Besides, it's not even true across the board for radioactive decay. A half-life that varies with concentration is the backbone of nuclear fission, for instance.
"The fool saith in his heart that there is no empty set. But if that were so, then the set of all such sets would be empty, and hence it would be the empty set." -- Wesley Salmon
- Vehrec
- Jedi Council Member
- Posts: 2204
- Joined: 2006-04-22 12:29pm
- Location: The Ohio State University
- Contact:
Re: Variable radioactive decay
The only reason chain-reaction half-lives are variable is because they destabilize isotopes by shooting neutrons into them and changing the mass, thereby disrupting the nucleus. 18% of all Neutron-U235 reactions withing nuclear reactors result in U236, so even that isn't totally reliable.
It isn't true that the half-life varies with concentration-the odds of spontaneous decay remain the same in all situations.
It isn't true that the half-life varies with concentration-the odds of spontaneous decay remain the same in all situations.
 Commander of the MFS Darwinian Selection Method (sexual)
Commander of the MFS Darwinian Selection Method (sexual)- Starglider
- Miles Dyson
- Posts: 8709
- Joined: 2007-04-05 09:44pm
- Location: Isle of Dogs
- Contact:
Re: Variable radioactive decay
This sounds like a misunderstanding of a real problem with radiocarbon dating, which is that the concentration of carbon 14 in the atmosphere varies over time (mostly in response to solar & cosmic radiation levels). Amusingly enough the various techniques used to correct for this would also correct for a non-logarithmic decay rate, if such a thing existed (which of course it doesn't).
- Kuroneko
- Jedi Council Member
- Posts: 2469
- Joined: 2003-03-13 03:10am
- Location: Fréchet space
- Contact:
Re: Variable radioactive decay
Under those conditions, it does. The context of the disputed statement made it clear that the claim was about the bulk half-life of a substance, which admittedly is different from the question in the OP.Vehrec wrote:It isn't true that the half-life varies with concentration...
That translates to constant bulk half-life only under the assumption that the individual atomic decays are independent, which can be false.Vehrec wrote:-the odds of spontaneous decay remain the same in all situations.
"The fool saith in his heart that there is no empty set. But if that were so, then the set of all such sets would be empty, and hence it would be the empty set." -- Wesley Salmon
Re: Variable radioactive decay
Wouldn't increasing the decay rates (were it possible) increase the amount of heat generated? Given numbers quoted like a million times, that could be a lot of heat.
>>Your head hurts.
>>Quaff painkillers
>>Your head no longer hurts.
>>Quaff painkillers
>>Your head no longer hurts.